Research
Computational, Affective &Translational neuroscience
The Computational Affective Translational Neuroscience group focuses on using computational modeling to harness the information provided by human neuroimaging data, with the view to applying these models for understanding affective processes, and particularly for pain perception and regulation. This group has a particular emphasis on naturalistic and computational neuroscience approaches, which seek to develop novel tasks and advanced computational methods to model the neural and behavioral dynamics in naturalistic environments.
(1) Affective Neuroscience (Associate Director Choong-Wan Woo)
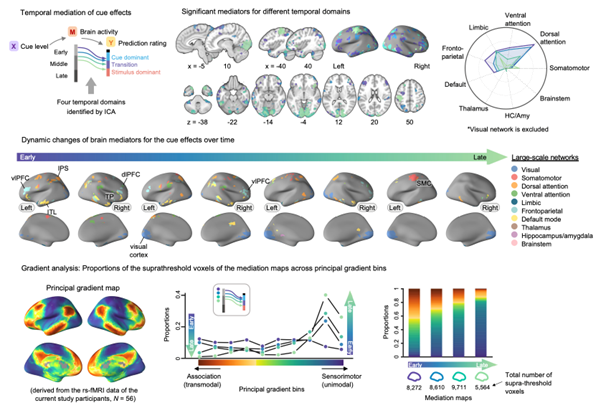
We examined the brain mechanisms of cue-induced pain modulation. Pain is not a mere reflection of noxious input. Rather, it is constructed through the dynamic integration of current predictions with incoming sensory input. However, the temporal dynamics of the behavioral and neural processes underpinning this integration remain elusive. In the current study involving 59 human participants, we identified a series of brain mediators that integrated cue-induced expectations with noxious inputs into ongoing pain predictions using a semicircular scale designed to capture rating trajectories. Temporal mediation analysis revealed that during the early-to-mid stages of integration, the frontoparietal and dorsal attention network regions, such as the lateral prefrontal, premotor, and parietal cortex, mediated the cue effects. Conversely, during the mid-to-late stages of integration, the somatomotor network regions mediated the effects of stimulus intensity, suggesting that the integration occurs along the cortical hierarchy from the association to sensorimotor brain systems. Our findings advance the understanding of how the brain integrates contextual and sensory information into pain experience over time.
(2) Naturalistic Neuroscience (Collaborating with Won Mok Shim, Michael Seng-Bum Yoo)
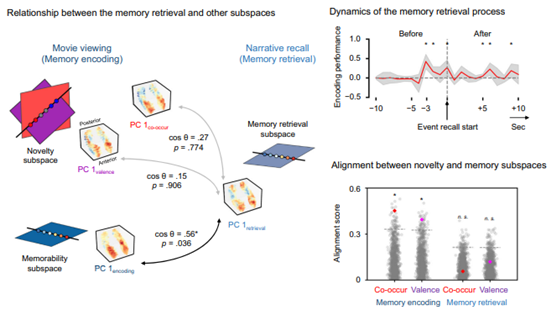
We examined the hippocampal mechanisms of memory retrival. Our naturalistic experiences are organized into memories through multiple processes, including novelty encoding, memory formation, and retrieval. However, the neural mechanisms coordinating these processes during real-world expreiences remain elusive. Using fMRI data acquired during movie viewing and subsequent narrative recall, we identified hippocampal neural subspaces associated with distinct memory processes and characterized their relationships. We developed metrics to quantify novelty in character co-occurrences and the valence of their relationships and estimate event memorability. Within the hippocampus, the subspaces encoding different types of novelty exhibit partial overlap, and these overlapping novelty subspaces align with the subspace involved in memorability. Notably, following event boundaries, hippocampal states within these subspaces align inversely along a shared coding axis, predicting subsequent recall performance. This novelty-memorability alignment is selectively observed during memory encoding but not during retrieval. Finally, the identified functional subspaces reflect the underlying intrinsic functional organization of the hippocampus. Our findings provide insights into how the hippocampus dynamically coordinates representations underlying memory encoding and retrieval at the population level to transform continuous naturalistic experiences into enduring memories that can be later accessed.
(3) Computational Neuroscience (Collaborating with Seok-Jun Hong)
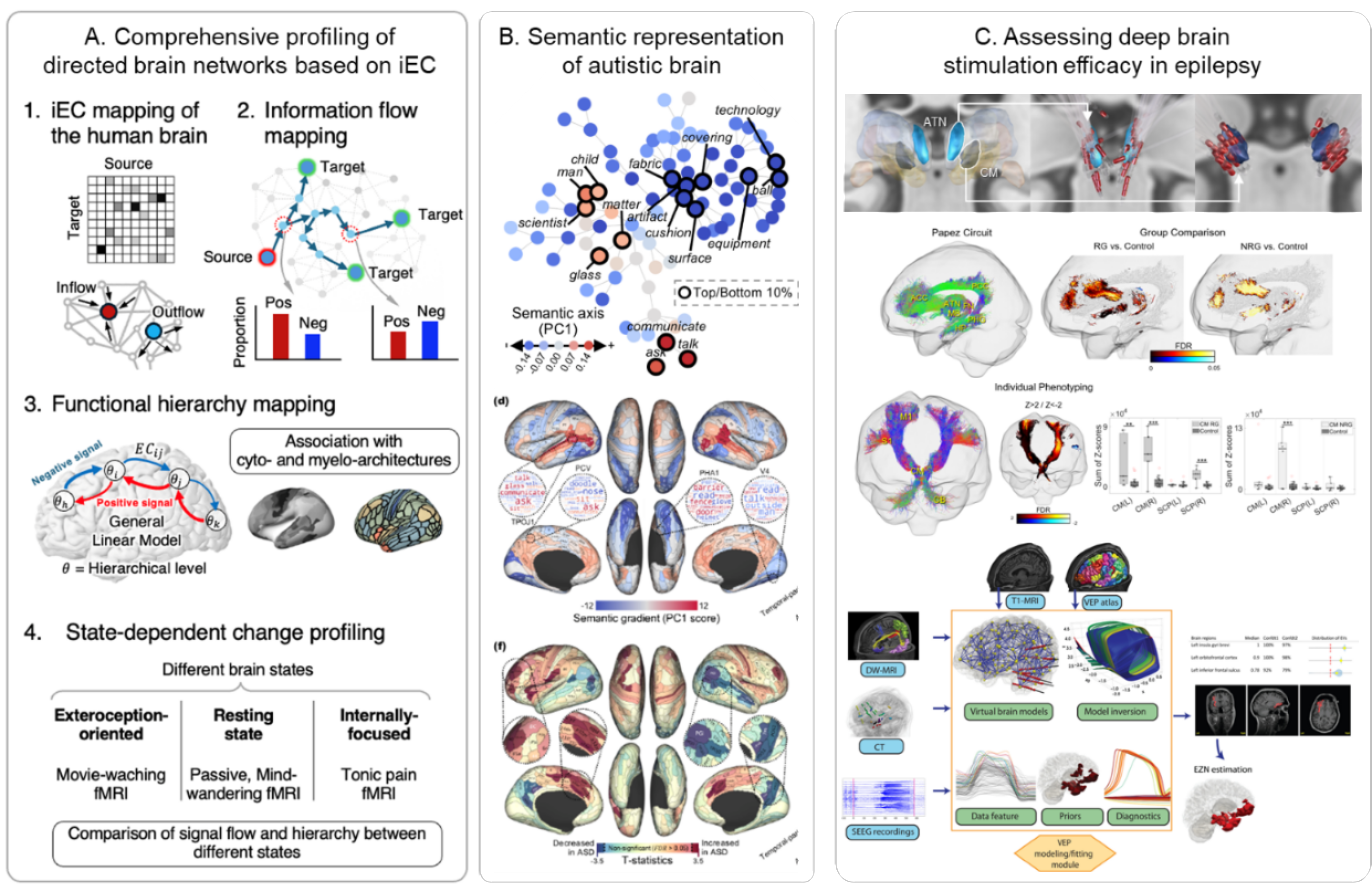
The one of our goal is to investigate organizational, developmental and neural dynamic principles of large-scale human brain networks. Learning these principles, we also aim to understand their abnormal process in clinical conditions, especially in developmental disorders such as autism and epilepsy. i) we have submitted to the journal the collaborative work entitled “In vivo cartography of state-dependent signal flow hierarchy in the human cerebral cortex” and now are in a revision process. ii) We finally published a developmental neuroscience study “A shifting role of thalamocortical connectivity in the emergence of cortical functional organization” in Nature Neuroscience. Iii) Moreover, we are at the final level to wrap the study of “Atypical whole-brain semantic representation in autistic children” to be submitted soon. iv) Finally, we have also investigated a network modeling approach in epilepsy, aiming at understanding the mechanism of how deep brain stimulation could reduce the seizure activity through synaptic plasticity.
(4) Machine Learning (Collaborating with Hyunjin Park)
Early detection of Alzheimer's disease (AD) is essential for timely management and consideration of therapeutic options; therefore, detecting the risk of conversion from mild cognitive impairment (MCI) to AD is crucial during neurodegenerative progression. Existing neuroimaging studies have mostly focused on group differences between individuals with MCI (or AD) and cognitively normal (CN), discarding the temporal information of conversion time. Here, we aimed to develop a prognostic model for AD conversion using functional connectivity (FC) and Cox regression suitable for conversion event modeling. We developed a prognostic model using a large-scale Alzheimer's Disease Neuroimaging Initiative dataset, and it was validated using external data obtained from the Open Access Series of Imaging Studies. We considered individuals who were initially CN or had MCI but progressed to AD and those with MCI with no progression to AD during the five-year follow-up period. As the exact conversion time to AD is unknown, we inferred this information using imputation approaches. We generated cortex-wide principal FC gradients using manifold learning techniques and computed subcortical-weighted manifold degrees from baseline functional magnetic resonance imaging data. A penalized Cox regression model with an elastic net penalty was adopted to define a risk score predicting the risk of conversion to AD, using FC gradients and clinical factors as regressors. Our prognostic model predicted the conversion risk and confirmed the role of imaging-derived manifolds in the conversion risk. The brain regions that largely contributed to predicting AD conversion were the heteromodal association and visual cortices, as well as the caudate and hippocampus. Our risk score based on Cox regression was consistent with the expected disease trajectories and correlated with positron emission tomography tracer uptake and symptom severity, reinforcing its clinical usefulness. Our findings were validated using an independent dataset. The cross-sectional application of our model showed a higher risk for AD than that for MCI, which correlated with symptom severity scores in the validation dataset. In sum, we proposed a prognostic model predicting the risk of conversion to AD. The associated risk score may provide insights for early intervention in individuals at risk of AD conversion.
SELECTED PUBLICATIONS
- Gim et al. (2024). Spatiotemporal integration of contextual and sensory information within the cortical hierarchy in human pain experience. PLoS Biology, 22(11), e3002910.
- Kim et al. (2024). A computational mechanism of cue-stimulus integration for pain in the brain. Science Advances, 10(37), eado8230.
- Kwon et al. (2025). Coordinated representations for naturalistic memory encoding and retrieval in hippocampal neural subspaces. Nature Communications, 16(1), 641
- Park et al. (2024). A shifting role of thalamocortical connectivity in the emergence of cortical functional organization. Nature Neuroscience, 27.1609-1619
- Kim et al., (2024). Prognostic model for predicting Alzheimer’s disease conversion using functional connectome manifolds. Alzheimer’s Research and Therapy, 16, 217.

